Talk about a scary chapter title: Biochemistry of the Molecules of Life. I don't even know if I want to keep on reading... But we're not going to let my lovely textbook's titles get the better of us.
Molecules: Organic and Not
The term Organic is used to define complex molecules (which would be all molecules) that are synthesized (a.k.a made) by life forms. Very few Organic molecules can be synthesized in laboratory settings. Most, if not all Organic Molecules contain Carbon and Hydrogen. There are four classes of organic molecules:
- Carbohydrates (also known as sugars)
- Lipids (also known as fats)
- Proteins
- Nucleic Acids (including DNA and RNA)
Organic Chemistry is the study of how exactly Carbon, Hydrogen, and Oxygen help maintain the life and energy cycle. My textbook tells me that an organic molecule begins with photosynthesis, which we will remember is a method used by Producers to create sugars using the energy of the sun. Because of this, Producers are the center point of all organic molecules.
During Photosynthesis, Producers fuse Carbon atoms extracted from the air to Oxygen and Hydrogen atoms extracted from water to build Carbohydrates. The carbohydrates are then converted by Producers and Consumers during cellular respiration into energy molecules called Adenosine Triphosphate (ATP for short. But just like with Deoxyribonucleic acid, we'll stick to the full name). The energy from these molecules of Adenosine Triphosphate are then used by the organism to fuel all of its chemical reactions. These chemical reactions are then used to create proteins, lipids, carbohydrates, and nucleic acids.
Inorganic is used to define any molecules that are not biological in origin. Normally, any molecule that does NOT include Carbon is defined by Scientists as Inorganic (remember that earlier we said most, if not all Organic Molecules contain Carbon). There are a few exceptions, but for the most part it is obvious which molecules are Inorganic by looking at their origin. Example: Diamonds contain Carbon molecules, however they are minerals and are obviously not organic.
It doesn't look like much, but the way the atoms are arranged structurally is important because it changes other properties of the molecules, both Physical and Chemical. Using Butane and Inobutane, it's interesting to note that while Butane boils at -1 degrees Celsius, Inobutane's boiling point is at -12 degrees Celsius.
Isomerization is the name used by Scientists to describe multiple molecules with the same molecular formula but different structural appearances (like Butane and Inobutane). In such cases, structural formulas (like the ones in our example) are used to given more information. A structural formula shows the bonds between the atoms of a molecule, preventing any confusion about which molecule is which. At a glance it looks kind of crazy, but in reality the formula is actually quite simple.
My textbook tells me that it should be noted that the structural formula of a molecule can be used for any type of molecule, organic or inorganic. So no one's left out here.
The Bohr model still contains more information, but it's so big and burdensome (and takes forever to fill out nicely) that it would be too difficult to write it out for every single molecule (especially since most organic molecules have several hundred atoms). The structural formula, however, is a nice way to get the need data quickly.
Inorganic is used to define any molecules that are not biological in origin. Normally, any molecule that does NOT include Carbon is defined by Scientists as Inorganic (remember that earlier we said most, if not all Organic Molecules contain Carbon). There are a few exceptions, but for the most part it is obvious which molecules are Inorganic by looking at their origin. Example: Diamonds contain Carbon molecules, however they are minerals and are obviously not organic.
Organic vs. Inorganic (a.k.a Here's Some More Information )
Organic molecules tend to be big in size with a large number of atoms.
vs
Inorganic molecules tend to be very small, with very few atoms involved.
- - - - - - -
Organic molecules are always bonded using the Covalent bond.
vs
Inorganic molecules usually bond using the Ionic Bond.
- - - - - - -
Organic molecules contain Carbon atoms.
vs
Inorganic molecules do not contain Carbon atoms (a few exceptions)
Molecular Formula and Isomers
Up until now, we've used molecular (or chemical) formulas to write down atoms "scientifically" (such as H2O and NaCl). Unfortunately, because Organic molecules have more complex combinations, the molecular formulas aren't specific enough for us to use when describing an individual molecule. The molecular formula only tells us the amount of atoms within a molecule, it doesn't include how the atoms are linked together. It'd be nice if we could just continue using this simple form of writing molecules, but if we did sooner or later we'd run into problems. Those problems are called Isomers.
Isomers are like identical twins in the molecular world. They share the same molecular formula, but are composed differently. My textbook gives the example of Butane and Isobutane. Both of these molecules have the formula of C3H10, but structurally they are quite different.
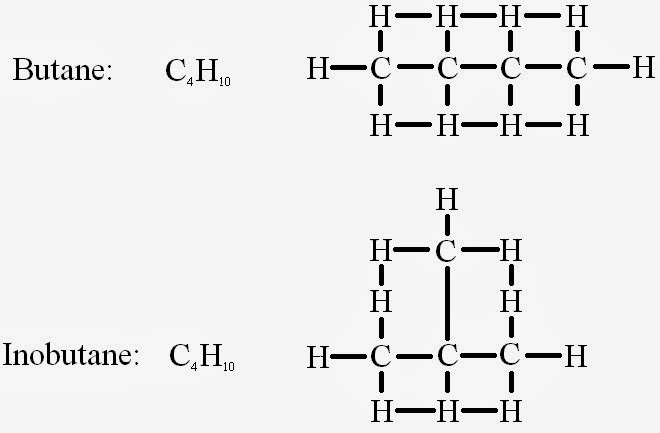 |
| Note the differences in appearance. |
Isomerization is the name used by Scientists to describe multiple molecules with the same molecular formula but different structural appearances (like Butane and Inobutane). In such cases, structural formulas (like the ones in our example) are used to given more information. A structural formula shows the bonds between the atoms of a molecule, preventing any confusion about which molecule is which. At a glance it looks kind of crazy, but in reality the formula is actually quite simple.
My textbook tells me that it should be noted that the structural formula of a molecule can be used for any type of molecule, organic or inorganic. So no one's left out here.
The Bohr model still contains more information, but it's so big and burdensome (and takes forever to fill out nicely) that it would be too difficult to write it out for every single molecule (especially since most organic molecules have several hundred atoms). The structural formula, however, is a nice way to get the need data quickly.
3D... Or Just 3 Dimensional
So far, we've been define atoms and molecules in 2d (using both the structural format and the Bohr model). In reality though, atoms aren't flat like the paper in my textbook or the screen on your computer/phone. If technology allowed us to make a diagram that showed what a single molecule really looks like, there would be atoms sticking out the screen towards you and away from you. With the way things have been going in the tech-world, we just might be able to in a couple of years, but today we’re out of luck.
It’s important for us to know the 3 dimensional nature of molecules because (like we learned from the previously) organic molecules are large, and the way they work often depends on how their atoms are arranged in their structure.
Bonding Time
In the diagram of Butane and Inobutane, the structural formula was shown in all its "bloom and glory" (whatever that means). The single lines you see between atoms are representations of single Covalent bonds connecting each atom in the molecule. This is a way to show that the atoms are sharing an electron pair. So we're done here... Ha, yeah right. There's more then one way to bring two atoms together *insert evil laugh here*.
When three lines are used between two bonded atoms, they are demonstrating a Triple Covalent bond. And, like with the others, the triple bond indicates that the atoms are sharing three pairs of electrons.
While both Ethene and Acetylene are flammable gasses, when exposed to heat Acetylene produces a flame much hotter then Ethene ever could. Why? It's because of the Triple bond. Triple bonds are harder to break then Double bonds, and likewise Double bonds are stronger then Single bonds. The strength of the bond effects the properties of the molecule. In Acetylene's case, the energy released when these bonds are broken attributes to the higher temperature in the fire.
Cool, right?
- Double Covalent Bonds
 |
| Meet Ethene (also called Ethylene). It's a colorless gas that's highly flammable and has a unpleasant, sweet smell and taste. |
When there are two lines drawn between atoms, it's called a Double Covalent bond. The double bond shows us that (in Ethene's case) the carbon atoms are sharing two pairs of electron.
- Triple Covalent Bonds
 |
| This is Acetylene. It's a colorless, flammable gas that is used for cutting and welding metals. |
While both Ethene and Acetylene are flammable gasses, when exposed to heat Acetylene produces a flame much hotter then Ethene ever could. Why? It's because of the Triple bond. Triple bonds are harder to break then Double bonds, and likewise Double bonds are stronger then Single bonds. The strength of the bond effects the properties of the molecule. In Acetylene's case, the energy released when these bonds are broken attributes to the higher temperature in the fire.
 |
| This is Acetylene in its prime. |
