It's not that this chapter is particularly long; there's just so much to cover in here (and it doesn't help that life doesn't take a break). Chemistry is so intense it needs its own subject, so to ask for a description of even the basics of Chemistry is a lot, right? So please don't be mad at me for settling this post so late. Please.
Molecules
Molecules are two or more atoms joined together, we know that. What we didn't know is that the reason atoms come together is because they're more stable then when their apart. Hmm, that's sort of like how it is with people. *Ahem* Back to atoms. The reason they're more stable is because when atoms come together, they're electron shells fill up (remember that an atom is stronger the more electrons it has in its shells). We already went over how the maximum number of the electrons in the outer electron shells is 8, and when atoms come together they most often fill the outer levels to the max. This is called the Octet rule. This isn't a rule made by the Scientists (despite how it might sound), it's more like a name given by the Scientist to classify something that nature does naturally.
NOTE: Hydrogen and Helium are special cases; their outer shells can only hold 2 electrons. In their case, the Octet Rule is changed to a Duet rule.
Bonding Time
So molecules are two or more atoms when they come together to fill their own electron shells, but that's just the over view. Science is all about getting down deep and understanding just how it happens. In this case "it" is called a Chemical Bond. Now obviously atoms don't bond the same way we do. They don't scheduled little "atom outings" where they get together and go shopping or bowling. A Chemical bond occurs when the "electrons in one atom interact with the electrons in another atom, causing the two atoms to 'stick' together."
As a way to write when a Chemical bond has occurred, scientists have developed the shorthand method of writing atoms after they've "bonded".
2, "H" is the Atomic Symbol (remember those?) for Hydrogen, and the plus sign shows that the two single Hydrogen atoms have come together through a Chemical Bond to form H2 (two Hydrogen atoms stuck together).
Okay, that's a really dull explanation (I mean: two Hydrogen atoms, Huzzah!), but basically it shows us how scientists write when a Chemical Bond has occurred
In the models above (called a Bohr model), two Chlorine atoms have bonded together. I've made it so we can clearly see where the bond has taken place. A Chlorine atom originally has 17 atoms (two in the third shell, 8 in the second shell, and 7 in the outer shell). Each Chlorine atom has just one electron less then the maximum electron in the outer shell. So when they come together, one electron from each atom attaches to the other's shell and fills it to the maximum complicity. Do we get it now?
As a way to write when a Chemical bond has occurred, scientists have developed the shorthand method of writing atoms after they've "bonded".
2, "H" is the Atomic Symbol (remember those?) for Hydrogen, and the plus sign shows that the two single Hydrogen atoms have come together through a Chemical Bond to form H2 (two Hydrogen atoms stuck together).
 |
| One Chlorine atom all alone. |
 |
| Two Chlorine atoms after a Chemical Bond. |
In the models above (called a Bohr model), two Chlorine atoms have bonded together. I've made it so we can clearly see where the bond has taken place. A Chlorine atom originally has 17 atoms (two in the third shell, 8 in the second shell, and 7 in the outer shell). Each Chlorine atom has just one electron less then the maximum electron in the outer shell. So when they come together, one electron from each atom attaches to the other's shell and fills it to the maximum complicity. Do we get it now?
Covalent and Ionic Bonds
There are actually two different versions of a Chemical Bond: Covalent and Ionic. Meaning that there are two different ways that electrons can be shared by atoms during a Chemical Bond.
Ionin means that the bond has a charge added to it. Looking back to the Covalent bonds, we'll remember that each atom is made up of protons, electrons and neutrons; protons have a positive charge, electrons have a negative charge, and neutrons have no charge. An Ion is a whole atom that has a charge, and this only happens when there are more protons then electrons, or vice versa. An atom with more protons then electrons has a positive charge and is known as a positive ion, while an atom with more electrons then protons has a negative charge and is labeled as a negative ion. Simple enough, right?
Covalent Bonds
The first bond, Covalent, is formed when the electrons shared during a Chemical Bond are equal. So for example, the Chlorine atoms above are in a Covalet bond because both atoms are gaining 1 more electron. Each Chlorine atom has 7 electrons in the outer shell, so when they share one atom with each other they suddenly have eight electrons, filling their outer shell and creating a Covalent bond. I'll put another example of a Covalent bond below. |
| Look at the lonely Hydrogen atom. Talk to the lonely Hydrogen atom. |
*sales person voice* But wait, there's more! Within the category of "Covalent bonds" there are two separate types: Polar and Non-Polar. Non-Polar Covalent Bonds form when there's equal sharing of the electrons (the Hydrogen atoms above are an example of a Non-Polar Covalent Bond). Each atom is sharing the electrons between them equally. Polar Covalent Bonds are formed when the electrons are being shared slightly unequally. Let's take H2O as our esteemed example.
In the Covalent bond for water, notice that despite the fact that all the atoms are still connected and still sharing each other's electrons, the electrons are more attracted to the Oxygen atom. Also note that the nucleus of the oxygen atom is quite a bit larger then the nucleus of both Hydrogen atoms. This is because the Oxygen as more protons in its nucleus (giving it a slightly positive charge) that "pulls" the electrons toward it (remember that electrons have a negative charge and opposites attract). Since the electrons are closer to the oxygen atom, the oxygen's side of the bond has a slightly negative charge (which would explain the subtraction a.k.a negative sign on the bottom of the figure). At the same time, the Hydrogen atoms are left with a positive charge because the electrons have left them (and now we know why there's a plus a.k.a positive sign near the top of the figure).
Both Non-Polar and Polar Covalent Bonds are much more stable then Ionic bonds because the atoms are actually attaching to each other through their electrons, instead of simply sticking together due to their charges. It takes a lot more strength to pull a Covalent bond apart then it does to pull apart an Ionic bond. According to my lovely textbook, there's no such thing as a "perfect" Covalent or "perfect" Ionic", so basically atoms are categorized by how familiar they are to one of these classifications, though they may have characteristics of both.
Ionic Bonds
Ionin means that the bond has a charge added to it. Looking back to the Covalent bonds, we'll remember that each atom is made up of protons, electrons and neutrons; protons have a positive charge, electrons have a negative charge, and neutrons have no charge. An Ion is a whole atom that has a charge, and this only happens when there are more protons then electrons, or vice versa. An atom with more protons then electrons has a positive charge and is known as a positive ion, while an atom with more electrons then protons has a negative charge and is labeled as a negative ion. Simple enough, right?
Since a negative ion and a positive ion have opposite charges (and opposites attract) they are attracted to one another. This attraction is strong enough to for a type of bond purely on their charges: Ionic. So basically instead of sharing electrons and attaching to each other, like in a Covalent Bond, they are simply sticking together. Like we put down before, Ionic bonds are much easier to break apart then Covalent Bonds.
Now my lovely textbook has decided to explain itself, or rather, explain why and how Ionic Bonds form. Sadly for us it's description is quite boggled down with lots of extra words, so let's help it out a little. :)
Now my lovely textbook has decided to explain itself, or rather, explain why and how Ionic Bonds form. Sadly for us it's description is quite boggled down with lots of extra words, so let's help it out a little. :)
How Do They Work?
By "they" we mean Ionic Bonds, and interestingly enough, it's not as simple as it might at first seem. Though my textbook tells me that we will be going over this again in Chemistry, I think that we should still understand it a bit more, right?
I'm going to use the atoms given as an example in my textbook for our own example. First question why do atoms form Ionic bonds? We know that in Covalent bonds their filling out their own electron shells, but it's different in Ionic bonds, right? Wrong. When forming an Ion (which is the first thing needed in an Ionic bond) atoms become more stable, and here's how:
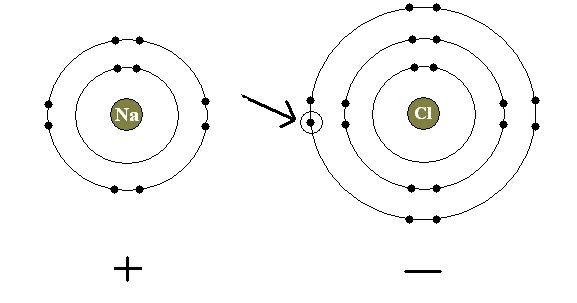
In Sodium Chloride (also known as table salt), the Sodium atom (with the atomic number of 11) has 2 electrons in the first electron shell (a.k.a energy level), 8 electrons in the second energy level (full), and only 1 in the third. On the other hand, the Chlorine atom (atomic number of 17) has its first and second energy levels completely full (so there are 8 electrons in each level, completing the Octet rule), and has only 1 space open for another electron in its third level.
When these two atoms are combined, this happens:
First the lonely electron from the Sodium atom leaves (or is donated) to the Chlorine atom, filling it's third shell and completing the Octet rule. Since Sodium is "donating" an electron, Scientists have labeled it as an electron donor. Since the Chlorine atom is accepting the generous gift of the Sodium atom, it's called an electron acceptor. Now due to this little exchange of electrons the Sodium atom has less electrons then protons, it has a positive charge (so it's now known as Mr. Sodium ion). Meanwhile the Chlorine atom, since it's gained an additional electron, now has a negative charge (and is now known as Ms.Chlorine ion). Of course, like we already know, opposites attract and in this case the opposite charges of the Sodium ion and the Chlorine ion are so strong that they create a bond themselves. That's the Ionic Bond. So really, unlike the Covalent Bond where electrons are being shared by both atoms and are attaching to each other, atoms with an Ionic bond are simply sticking together due to their charges.
So why do atoms like to use the Ionic Bond? The answer: because it makes them more stable. With the Chloride atom it's easy to see that the Ionic Bond is making it stronger because its third shell is completely full. But how can it make the Sodium atom stable when it's loosing an electron? The logic behind this actually makes sense! When the lonely electron in Sodium's outer shell goes to the Chlorine atom's outer shell, Sodium's third shell disappears with it. So now Sodium is left with a full outer shell (the second shell), get it? My textbook says that we shouldn't get boggled down with this because we'll be going over this in Chemistry, so no worries if we don't get it right now.
When it comes to breaking apart Ionic bonds, here's a simple example. Who's ever put salt in water before? Maybe it's not a hobby, but for the most part I think we all have either when we're cooking or doing a science experiment. When combined with water, salt (a.k.a Sodium Chloride) breaks up and dissolves, right? The reason is because the water is breaking apart (or dissociating) the Ionic bonds between the Sodium atoms and the Chlorine atoms, leaving them once again as lonely Na and Cl atoms. (*sniff*)
Chemically, this is written as:
That's all I'm putting for part 3, but part 4's coming soon (doesn't that sound like a movie review?).
I'm going to use the atoms given as an example in my textbook for our own example. First question why do atoms form Ionic bonds? We know that in Covalent bonds their filling out their own electron shells, but it's different in Ionic bonds, right? Wrong. When forming an Ion (which is the first thing needed in an Ionic bond) atoms become more stable, and here's how:
In Sodium Chloride (also known as table salt), the Sodium atom (with the atomic number of 11) has 2 electrons in the first electron shell (a.k.a energy level), 8 electrons in the second energy level (full), and only 1 in the third. On the other hand, the Chlorine atom (atomic number of 17) has its first and second energy levels completely full (so there are 8 electrons in each level, completing the Octet rule), and has only 1 space open for another electron in its third level.
When these two atoms are combined, this happens:
First the lonely electron from the Sodium atom leaves (or is donated) to the Chlorine atom, filling it's third shell and completing the Octet rule. Since Sodium is "donating" an electron, Scientists have labeled it as an electron donor. Since the Chlorine atom is accepting the generous gift of the Sodium atom, it's called an electron acceptor. Now due to this little exchange of electrons the Sodium atom has less electrons then protons, it has a positive charge (so it's now known as Mr. Sodium ion). Meanwhile the Chlorine atom, since it's gained an additional electron, now has a negative charge (and is now known as Ms.Chlorine ion). Of course, like we already know, opposites attract and in this case the opposite charges of the Sodium ion and the Chlorine ion are so strong that they create a bond themselves. That's the Ionic Bond. So really, unlike the Covalent Bond where electrons are being shared by both atoms and are attaching to each other, atoms with an Ionic bond are simply sticking together due to their charges.
So why do atoms like to use the Ionic Bond? The answer: because it makes them more stable. With the Chloride atom it's easy to see that the Ionic Bond is making it stronger because its third shell is completely full. But how can it make the Sodium atom stable when it's loosing an electron? The logic behind this actually makes sense! When the lonely electron in Sodium's outer shell goes to the Chlorine atom's outer shell, Sodium's third shell disappears with it. So now Sodium is left with a full outer shell (the second shell), get it? My textbook says that we shouldn't get boggled down with this because we'll be going over this in Chemistry, so no worries if we don't get it right now.
When it comes to breaking apart Ionic bonds, here's a simple example. Who's ever put salt in water before? Maybe it's not a hobby, but for the most part I think we all have either when we're cooking or doing a science experiment. When combined with water, salt (a.k.a Sodium Chloride) breaks up and dissolves, right? The reason is because the water is breaking apart (or dissociating) the Ionic bonds between the Sodium atoms and the Chlorine atoms, leaving them once again as lonely Na and Cl atoms. (*sniff*)
Chemically, this is written as:
 |

.bmp)