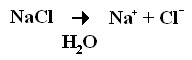Well, we've come far! ( < that was sarcasm by the way). Actually, we're almost done with this chapter, so all in all we have come far. Kind of.
This part of the chapter starts off by describing the equation given in the previous lesson. In that distant lesson we went over Covalent and Ionic bonds, and this equation shows what happens when Sodium Chloride (the NaCl on the left) mixes with water (the H2O). In review, the water breaks apart the Ionic bond that was keeping the Sodium and Chlorine atoms together and we're left with lonely, separate Na and Cl atoms. When using this Chemical equation, Scientist use the terms Reactants and Products to distinguish the different parts of the equation. The molecules/atoms on the left side (in this case NaCl) are referred to as the Reactants, while the atoms that make them up (Na and Cl separately) are called the End Products or simply Products.
Now we know how scientists love to label things, so here's a couple more labels for us. When looking at a solution, there are at least two different molecules mixing together. The Solvent is what scientists call the group of molecules that make up the bigger part of the solution (in this case the water), while the Solute is the group of molecules that make up the small part of the solution (in this case the NaCl). This is just to better organize the make up of life around us.
The reason this is important? Well, most, if not all, organisms live within solution environments. We living in a world where the air is a solution of oxygen, hydrogen, ozone, and a good many other molecules. Fish live in a world where the water is a solution of either H2O and NaCl, or H2O and whatever it is that lies in fresh water. If that's not enough to prove that this is important then: my textbook tells me it's important to understand about solutions so *ahem* it's important!
In most solutions, the solvent is water, but the concentration of hydrogen ion, H+, and hydroxide ions, OH+, varies. These ions naturally build up within water, even if it's "pure" H2O, and the ratio of which one is the solvent and which is the solute changes the acidity of the solution. A solution that has more H+ ions then OH- ions is an acid, while a solution with more OH- then H+ is a base. The more H+ ions there are the stronger the acid, and likewise the more OH- there are the more basic the solution is.
Look a pH scale! Okay, now what is a pH scale? Simply speaking, it's a chart that allows scientists to organize the acidity of any given solution. Unlike most scales, this one doesn't start at 0, it starts at 7. 7 is the considered "neutral" in the pH Scale. This means that pure water has an equal amount if H+ ions and OH- ions and is neither acidic nor basic. It's right, smack in the middle. Oh look, blood is there too, so that means it must also be a neutral solution.
Now, in the pH scale you can go either up or down. The higher you are in the scale, the lower your number is and the more acidic you are. The lower you are on the scale, the higher you are in numbers and the more basic you are.
NOTE: My lovely textbook tells me to be aware that sometimes basic solutions are called alkaline solutions.
Reactants and Products
Law of Conservation of Mass
In the equation given above, the number of Reacting atoms equal the same amount of atoms in the End Product. This balance of molecules is called the Law of Conservation of Mass. Scientist love to label things, especially things that are always true in every circumstance.
The Law of Conversation of Mass means that no matter what, you will always have the same number of atoms at the end as you did in the beginning. A random atom (or molecule) can't just appear out of no where. Okay, so right now we're probably all thinking "well obviously you're going to have the same amount of atoms as when you started out". The reason this is used in Chemistry is because, by knowing that this is a Law and therefore can't change, scientists can figure just how many molecules can be made from a given number of products.
The Law of Conversation of Mass means that no matter what, you will always have the same number of atoms at the end as you did in the beginning. A random atom (or molecule) can't just appear out of no where. Okay, so right now we're probably all thinking "well obviously you're going to have the same amount of atoms as when you started out". The reason this is used in Chemistry is because, by knowing that this is a Law and therefore can't change, scientists can figure just how many molecules can be made from a given number of products.
For example we'll take Sodium Chloride again. We know that there needs to be one Sodium atom and one Chlorine atom to make the Reactant Sodium Chloride. Say a group of 6 Sodium ions and a group of 6 Chlorine ions come together. How many Sodium Chloride molecules will be created? . . . If we all said 3 then we're all right (give yourself a pat on the back). Now, how did we know that 6 Sodium atoms and 6 Chlorine atoms would make 3 Sodium Chloride atoms? Why not 4, or 7? The answer is the Law of Conservation of Mass. We know that, no matter what, there will always be 6 Sodium atoms and 6 Chlorine atoms and when combining one of each with each other we get three pairs - 3 NaCl. New atoms could not suddenly appear and make more Sodium Chloride molecules, nor could any of the atoms we already had disappear suddenly.
Water (duh, Duh, DUH)
This part of the lesson really takes us in a different direction. I mean, we have Reactants and Products, and the Law of Conservation of Mass, and then (all of a sudden) we have water. Talk about unexpected.
According to my lovely textbook, it's important for us to understand the molecular structure of water because it's a key role in most if not all organisms. 70%-80% of the matter of any organism is water (us included). The reason is because of how water is made up.
We remember that, in the previous lesson, we went over how H2O is formed by Covalent Polar Bonds, and that the result is an atom with a slightly negative charge on the Oxygen side and a slightly positive charge on the Hydrogen side (note that it is not an ionic bond though). What we didn't go over what that, because of this unique structure, the water atom ends up being very compatible with many different types of atoms (including salts, sugars, and proteins).
Also, the unique polarity of H2O allows it to form a completely new type of bond: a Hydrogen Bond. Because the electrons shade towards the oxygen atom, and therefore the oxygen has a slightly negative charge, it attracts other slightly positive hydrogen atoms from other water molecules. Scientists refer to this as a Hydrogen bond most likely because, when you see a cluster of water molecules, the Hydrogen bond is what keeps them together.
"Another property of water that allows for hydrogen bonding is the actual structure of the molecule itself." Notice that in the model above the water molecule isn't a straight line of atoms; it looks more like a "Mickey Mouse" head (we take this time to thank Walt Disney for inventing Mickey Mouse, for without him we'd be at a loss as to how to describe the structure of a water molecule *moment of silence*).
*Ahem* Back to H2O. The water molecules structure as a "Mickey Mouse head" allows other water molecules to fit in the open spaces tightly, like so:
"Another property of water that allows for hydrogen bonding is the actual structure of the molecule itself." Notice that in the model above the water molecule isn't a straight line of atoms; it looks more like a "Mickey Mouse" head (we take this time to thank Walt Disney for inventing Mickey Mouse, for without him we'd be at a loss as to how to describe the structure of a water molecule *moment of silence*).
*Ahem* Back to H2O. The water molecules structure as a "Mickey Mouse head" allows other water molecules to fit in the open spaces tightly, like so:
 |
| This special structure allows the Hydrogen bond to be much stronger then it would be normally. |
According to my textbook, the final property of water that makes them special is that that the temperature of water rises and falls very slowly (in nature). This allows organisms to obtain homeostasis.
Speaking of water, did you know that it's a solution? Well, yes, it's a solution to droughts and thirst and how to grow a good vegetable garden, but we're pretty sure that's not what the textbook meant. A Solution is a homogeneous combination of atoms, whether in the form of a liquid, solid, or gas. Homogeneous means the "same throughout". Here's an example: ocean water is a homogeneous solution of NaCl (which we'll remember is Sodium Chloride, a.k.a salt) and H2O molecules. Even though they are mixed together, the atoms are still the same (hence the "homogeneous").Solutions, Solvents, and Solutes
Now we know how scientists love to label things, so here's a couple more labels for us. When looking at a solution, there are at least two different molecules mixing together. The Solvent is what scientists call the group of molecules that make up the bigger part of the solution (in this case the water), while the Solute is the group of molecules that make up the small part of the solution (in this case the NaCl). This is just to better organize the make up of life around us.
The reason this is important? Well, most, if not all, organisms live within solution environments. We living in a world where the air is a solution of oxygen, hydrogen, ozone, and a good many other molecules. Fish live in a world where the water is a solution of either H2O and NaCl, or H2O and whatever it is that lies in fresh water. If that's not enough to prove that this is important then: my textbook tells me it's important to understand about solutions so *ahem* it's important!
Acids and Bases and Porcelain Vases
Alright, that title was obviously just a joke. I mean: Acids and Bases no, but learning about Porcelain Vases in science? More importantly Biology? I don't think so. Now, about Acids and Bases.In most solutions, the solvent is water, but the concentration of hydrogen ion, H+, and hydroxide ions, OH+, varies. These ions naturally build up within water, even if it's "pure" H2O, and the ratio of which one is the solvent and which is the solute changes the acidity of the solution. A solution that has more H+ ions then OH- ions is an acid, while a solution with more OH- then H+ is a base. The more H+ ions there are the stronger the acid, and likewise the more OH- there are the more basic the solution is.
pH Scale
Now, in the pH scale you can go either up or down. The higher you are in the scale, the lower your number is and the more acidic you are. The lower you are on the scale, the higher you are in numbers and the more basic you are.
NOTE: My lovely textbook tells me to be aware that sometimes basic solutions are called alkaline solutions.
Hey, a Porcelain Vase! ... I just couldn't resist.
Indicators
Scientists can add various liquids to a solution in order to tell whether it's an acid or a base. These liquids are called indicators and are usually chemicals that change color in a solution. The scientists use the color to determine the number of a solution on the pH scale.
This is the end of Chapter 2! *gasps in relief and goes to make lunch and read some more from biology textbook*
This is the end of Chapter 2! *gasps in relief and goes to make lunch and read some more from biology textbook*